Redox Biochemistry
We study biomolecular reactions that convert electrochemical energy into chemical bonds of reduced products. This research advances the development of enzyme-based and microbial-based systems for the production of energy compounds and carriers.
Featured Publications
[FeFe]- and [NiFe]-Hydrogenase Diversity, Mechanism, and Maturation, Biochimica et Biophysica Acta - Molecular Cell Research (2015)
[FeFe]-Hydrogenase Oxygen Inactivation Is Initiated by the Modification and Degradation of the H Cluster 2Fe Subcluster, Journal of the American Chemical Society (2015)
View all NREL physical biochemistry publications.
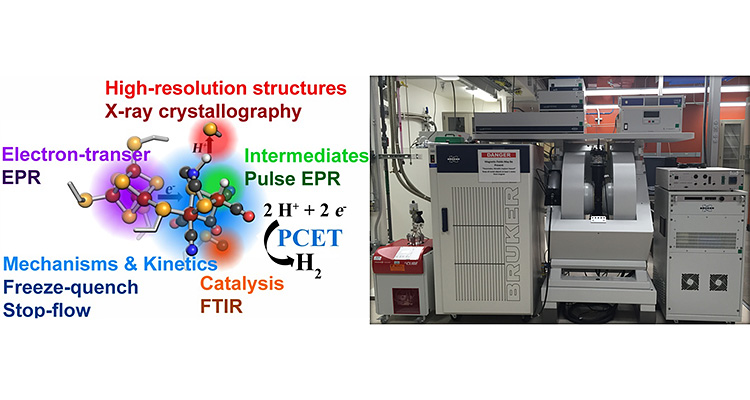
Biophysics
To evaluate the paramagnetic properties of redox active cofactors in metallo- and flavoproteins we have a spin resonance facility that houses a Bruker E-500 EPR and a E-580 pulse EPR with closed-cycle, He-cryostats (temperature range >4K), and a fiber optic laser and optical electron paramagnetic resonance (EPR) probes.
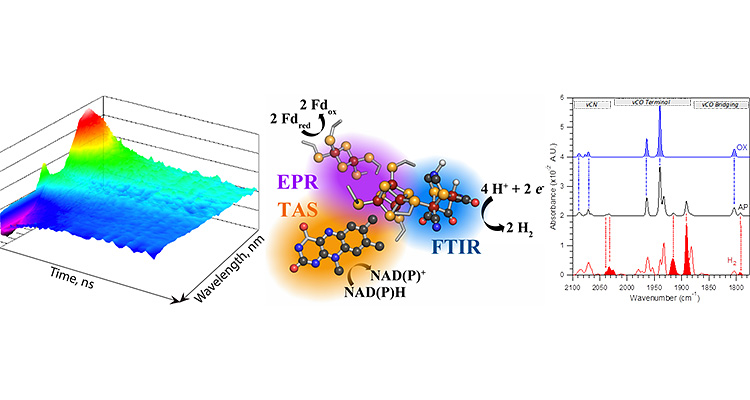
Optical Spectroscopy
Our capabilities include a range of infrared, mid-infrared, and ultrafast transient absorption (UV/visible) spectrometers that facilitate the study of very fast electron transfer events in a variety of redox active cofactors.
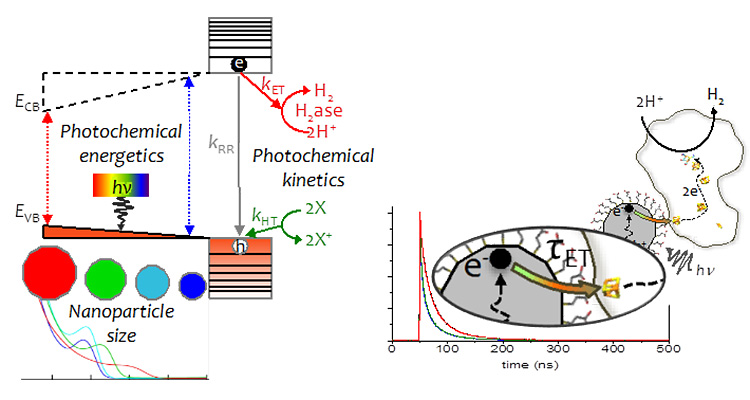
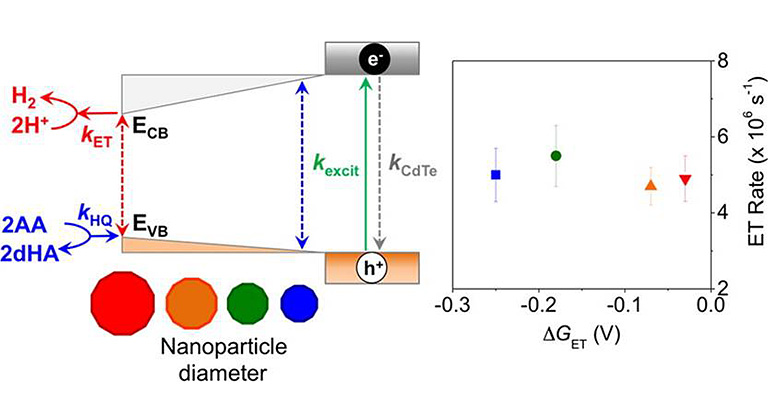
Photobiohybrids
We have developed enzyme-nanoparticle complexes as photochemical systems to couple light harvesting to drive electron conversion reactions for deciphering fundamental mechanisms of electron transfer and catalysis in redox enzymes.

Anaerobic Biochemistry
We have developed recombinant expression systems for the production of a range of different metalloproteins including hydrogenases, hydrogenase maturases, ferredoxins, and flavin-containing enzymes.
Research Team
Principal Investigators
Pin-Ching Maness
Principal Scientist, Photobiology Group Manager
PinChing.Maness@nrel.gov303-384-6114
Rachel Ward (University of Colorado Boulder)
Collaborators
Arizona State University, Jones Research Group
Carnegie Mellon University, Guo Research Group
Montana State University, Bothner Lab
Montana State University, Broderick Research Group
University of Colorado Boulder, Dukovic Research Group
University of Colorado Boulder, Smalyukh Research Group
University of Georgia Athens, Adams Research Group
University of Kentucky, Miller Research Group
Utah State University, Seefeldt Research Group
Funding for NREL's Redox Biochemistry R&D is provided by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program, and the Biological and Electron Transfer and Catalysis EFRC, an Energy Frontiers Research Center funded by the U.S. Department of Energy, Office of Science.
Share

![Illustration of an H-cluster and the conserved proton-transfer pathway (labeled with an arrow as PT) in [FeFe]-hydrogenase. A cartoon of a grey blob represents the structure with surface representations of blue spirals and helixes. An inset illustration shows a close-up of the surface where green tube-shaped structures represent carbon, red short tubes represent oxygen, blue spirals and helixes represent nitrogen, orange short tubes represent sulfur, and rust spheres represent iron. The black arrows depict the proton-transfer pathway for the OC-NC-Fe-CO-CN-S-N-H cluster to Cys169 to Glu141 to Ser189 and Glu144.](/bioenergy/assets/images/redoxbiochem1.jpg)
![Side by side illustrations of X-ray structures of binuclear cofactors of the hydrogenase catalytic sites. (A) shows the NiFe cluster of [NiFe(Se)]-hydrogenases with yellow, brown, green, pink, and blue spheres attached to tubes of white, green, blue, and brown, and surrounded by green ribbon/helixes. This diagram also has labels for Glu 18, Arg 463, Ser 486, and His 72. (B) shows the 2Fe subcluster of the [FeFe]-hydrogenase H cluster with yellow, brown, grey, red and blue spheres attached to tubes of grey, yellow, and brown, surrounded by blue ribbon/helixes. This diagram also has labels for Ser 232, Met 353, Met 497, Cys 299, Phe 417, and Lys 358. The H-bonding interactions (dashed lines, black font) and conserved, exchangeable groups (dashed lines, purple font) are labeled. Ni, nickel; Se, selenium; Fe, iron.](/bioenergy/assets/images/redoxbiochem3.jpg)


