
Susan Habas
Senior Scientist, Nanoscience & Materials Chemistry
Susan.Habas@nrel.gov
303-384-6734
 https://orcid.org/0000-0002-3893-8454
https://orcid.org/0000-0002-3893-8454
Google Scholar
Research Interests
-
Design, synthesis, and characterization of nanostructured catalysts
-
Controlled surface chemistry for selective chemical transformations
-
Scalable methods for solution-phase nanomaterials synthesis
-
Production of premium fuels and chemicals from biomass
Affiliated Research Programs
-
Heterogeneous Catalysis for Thermochemical Conversion
Education
-
Ph.D., Chemistry, University of California at Berkeley, 2003–2008
-
Fulbright Scholar, Massey University, New Zealand, 2002–2003
-
A.B., Chemistry/Biochemistry, Wheaton College, 1997–2001
Professional Experience
-
Senior Scientist, National Renewable Energy Laboratory (NREL), National Bioenergy Center (NBC), 2014–present
-
Staff Scientist, NREL, NBC, 2013–2014
-
Staff Scientist, NREL, National Center for Photovoltaics (NCPV), 2012–2013
-
Postdoctoral Researcher, NREL, NCPV, 2009–2012
-
Postdoctoral Researcher, Lawrence Berkeley National Laboratory, Molecular Foundry, 2008–2009
Patents
-
"Metal Phosphide Catalysts and Methods for Making the Same and Uses Thereof," U.S. Patent No. 9,636,664 B1 (2017)
-
"Metal Phosphide Catalysts and Methods for Making the Same and Uses Thereof," U.S. Patent Application No. 2017/0197200 Al (2017)
-
"Catalysts and Methods for Converting Biomass to Liquid Fuels," U.S. Provisional Patent Application 62/414,496 (2016)
Featured Publications
-
"Transitioning Rationally Designed Catalytic Materials to Real 'Working' Catalysts Produced at Commercial Scale: Nanoparticle Materials," Catalysis (2017)
-
"An Investigation Into Support Cooperativity for the Deoxygenation of Guaiacol Over Nanoparticle Ni and Rh2P," Catalysis Science & Technology (2017)
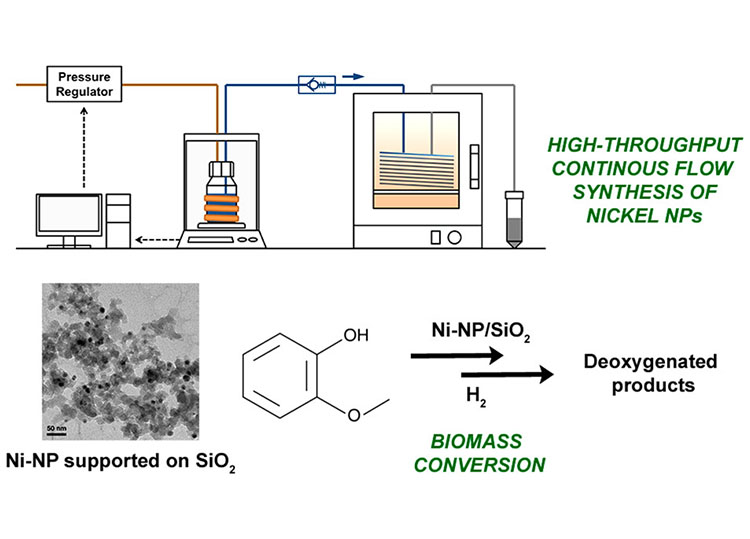
- "High-Throughput Continuous Flow Synthesis of Nickel Nanoparticles for the Catalytic
Hydrodeoxygenation of Guaiacol," Chemistry of Materials (2016)
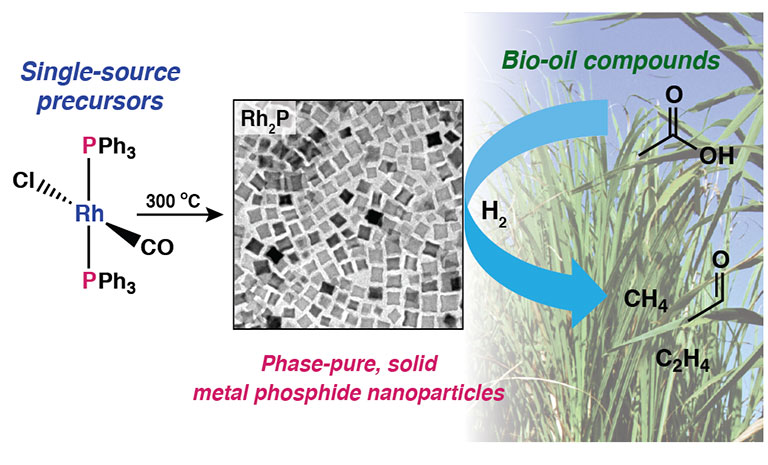
- "A Facile Molecular Precursor Route to Metal Phosphide Nanoparticles and Their Evaluation
as Hydrodeoxygenation Catalysts," Chemistry of Materials (2016)
-
"Conversion of Dimethyl Ether to 2,2,3-trimethylbutane over a Cu/BEA Catalyst: Role of Cu Sites in Hydrogen Incorporation," ACS Catalysis (2015)

-
"Surface Chemistry Exchange of Alloyed Germanium Nanocrystals: A Pathway Toward Conductive Group IV Nanocrystal Films," Journal of Physical Chemistry Letters (2013)
-
"Low-Cost Inorganic Solar Cells: From Ink to Printed Device," Chemical Reviews (2010)
-
"Influence of Size, Shape, and Surface Coating on the Stability of Aqueous Suspensions of CdSe Nanoparticles," Chemistry of Materials (2010)
-
"Probing Compositional Variation Within Hybrid Nanostructures," ACS Nano (2009)
-
"Interfacing Metal Nanoparticles with Semiconductor Nanowires," Chemistry of Materials (2009)
-
"Localized Pd Overgrowth on Cubic Pt Nanocrystals for Enhanced Electrocatalytic Oxidation of Formic Acid," Journal of the American Chemical Society (2008)
-
"Shape Control of Colloidal Metal Nanocrystals," Small (2008)
-
"Selective Growth of Metal and Binary Metal Tips on CdS Nanorods," Journal of the American Chemical Society (2008)
-
"Shaping Binary Metal Nanocrystals through Epitaxial Seeded Growth," Nature Materials (2007)
-
"Morphological Control of Catalytically Active Platinum Nanocrystals," Angewandte Chemie International Edition (2006)
View all NREL Publications for Susan Habas.
Share

