
Jack Ferrell
Research Engineer
Jack.Ferrell@nrel.gov
303-384-7777
 https://orcid.org/0000-0003-3041-8742
https://orcid.org/0000-0003-3041-8742
Google Scholar
Research Interests
Jack Ferrell manages tasks on CO2 utilization and on analytical standardization for pyrolysis oil. Research interests include:
- CO2 utilization: conversion of CO2 into value-added products by both electrochemical and thermochemical pathways
- Standardization of analytical techniques for the analysis of bio-oils
-
Electrochemical upgrading of biomass-derived intermediates
CO2 Utilization
CO2 is a pervasive waste stream, and is commonly released into the atmosphere. Certain industrial processes, such as ethanol biorefineries, produce a concentrated CO2 waste stream which is captured. Biorefineries currently sell this CO2 to the food and beverage industry. The recent availability of inexpensive intermittent electricity produced from renewables is changing the energy landscape, and the amount of this electricity is projected to increase in coming years. This has led to an opportunity to use this electricity for the conversion of CO2. Conversion of this CO2 into more valuable products would enhance the economic viability of biorefineries.
Starting in 2017, I began leading a new CO2 Utilization project investigating both electrochemical and thermochemical conversion pathways. Specifically, we are interested in low-temperature electrochemical conversion, with a focus on catalyst development and the development of a practical CO2 electrolyzer (based on a membrane-electrode-assembly (MEA)). To date, only Cu-based catalysts have been able to produce C2+ hydrocarbon products at appreciable rates. New catalysts are needed that can achieve both higher current densities and better selectivity to desired products. Additionally, no consensus exists today on what a low-temperature CO2 electrolyzer should look like, as there is no agreement on the proper MEA architecture to ensure stable and long-term operation. We are investigating different MEA architectures to enable stable and extended operation with gas-phase CO2.
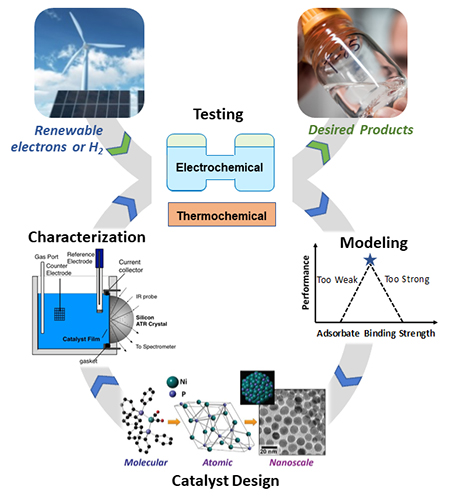
In thermochemical conversion, we are focused on catalyst development to allow for process intensification, where CO2 can be directly converted to desired products in a single reactor. Multifunctional catalysts are needed, as thermochemical CO2 utilization has typically required a reactor to first produce syngas, followed by a second reactor to convert syngas into desired products. To combine these steps into a single reactor, catalysts must be developed that combine functionalities of both steps. For thermochemical conversion, use of renewable electricity is integrated via the electrolysis of water, producing hydrogen to be used in the conversion of CO2.
Standardization of Analytical Techniques for Analysis of Bio-Oils
In order to economically produce renewable fuels and chemicals from biomass, the biomass must first be deconstructed, and then processed further (upgraded) to produce either a finished product, or an intermediate stream that can be sent to a refinery. Pyrolysis is a promising thermochemical route for deconstructing the biomass feedstock. Pyrolysis, typically run around 500 ⁰C, produces pyrolysis vapors which are often condensed to form pyrolysis oil, or bio-oil. Bio-oil is an extremely complex mixture, containing several hundred compounds, and a large variety of chemical functionalities.

To inform both pyrolysis and upgrading processes, reliable analytics are needed for bio-oil. A collaborative project with Pacific Northwest National Laboratory began in 2014 to standardize analytical techniques for analysis of bio-oils, and Oak Ridge National Laboratory joined the project in 2015. Development of standard quantitative methods, and subsequent method validation via Round Robin, are ongoing. As of August 2015, the following standard methods have been developed for bio-oil analysis:
- GC/MS for analysis of volatile components
- Titration for carboxylic acid content (also known as CAN/TAN analysis)
- Titration for carbonyl content
- 31P NMR for hydroxyl group quantification
- HPLC for carboxylic acids
- HPLC for carbonyl compounds
- 13C NMR for carbon functional groups.
Methods that prove reliable (via Round Robin) will be published as Laboratory Analytical Procedures (LAPs). Many LAPs have been developed for biomass analysis, and we plan to add methods for bio-oils to this list. As of August 2015, our first Round Robin is not yet complete, and thus zero bio-oil LAPs have been published. For updates, check here, the LAP website, or contact me directly.
Electrochemical Upgrading of Biomass-Derived Intermediates
Compared to petroleum-based fuels, biomass-derived intermediates (e.g., pyrolysis
vapors) are hydrogen-deficient and oxygen-rich, and require upgrading (hydrogenation
and deoxygenation) to produce fuels and chemicals. Current upgrading methods have
been adapted from the petroleum industry, and have significant limitations for processing
biomass-intermediates (e.g., catalyst deactivation and use of large excesses of H2).
I’m interested in developing new processes for biomass upgrading, and I feel that
electrochemical technologies are very promising for this application. Electrochemical
upgrading offers several advantages:
- The driving force for reaction (applied voltage) is easily controlled, and can be used to control reaction rates and selectivities.
- Efficient hydrogen utilization.
- Work to-date has shown that electrochemical upgrading produces a drastically different product slate than existing thermochemical methods.
- Excess renewable electricity (from wind or solar) can be used to produce storable fuels and chemicals.
- Electrochemical systems are modular and easily scalable.
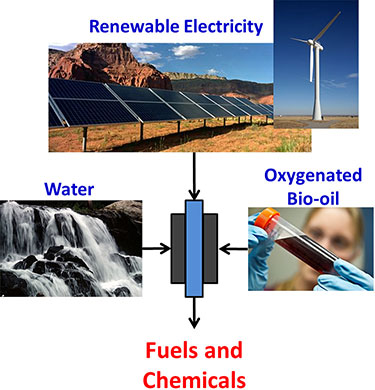
I’m interested in partnering with companies or institutions to develop electrochemical technology for processing biomass-intermediates. I have interests and experience with both low temperature (polymer membrane) and intermediate temperature (ceramic membrane) systems. Please contact me with further questions.
Affiliated Research Programs
-
Biomass Characterization (PI)
-
Heterogeneous Catalysis for Thermochemical Conversion (PI)
-
Thermochemical Process Integration, Scale-up, and Piloting (collaborator)
-
Biomass Feedstocks (collaborator)
Areas of Expertise
-
Analytical chemistry
-
Gas chromatography
-
Liquid chromatography
-
Mass spectrometry
-
Titrations
-
Nuclear magnetic resonance
-
Thermal gravimetric analysis
-
-
Electrochemical technologies
-
Low temperature: solid polymer electrolytes, both proton-conducting and alkaline-exchange membranes
-
Intermediate and high temperature: both proton-conducting and oxygen-anion-conducting ceramic membranes
-
-
Heterogeneous catalysis
-
Gas to liquids
-
Kinetics and reaction mechanisms
-
Physical and chemical characterization
-
Metal sulfides
-
Polyoxometallates
-
-
Reactor scale-up and validation
Education
-
Ph.D., Chemical Engineering, Colorado School of Mines, 2004–2009
-
B.S., Chemical Engineering, University of Virginia, 2000–2004
Professional Experience
-
Staff Engineer, Thermochemical Catalysis R&D, National Renewable Energy Laboratory (NREL), National Bioenergy Center (NBC), 2012–present
-
Temporary Engineer, Thermochemical Catalysis R&D, NREL, NBC, 2011–2012
-
Postdoctoral Associate, National Energy Technology Laboratory (NETL), Energy Systems Dynamics and Separations & Fuels Processing Divisions, 2010–2011
Accreditations
-
American Institute of Chemical Engineers (AIChE)
-
The Electrochemical Society (ECS)
- American Chemical Society (ACS)
Patents
-
"Immobilized Heteropoly Acids and the Use of the Same for Electrode Stabilization and Enhancement," U.S. Patent No. 8,753,997 (2014)
Featured Publications
- "Characterization of Upgraded Fast Pyrolysis Oak Oil Distillate Fractions from Sulfided and Non-Sulfided Catalytic Hydrotreating," Fuel (2017)
-
"Quantitative 13C NMR Characterization of Fast Pyrolysis Oils," RSC Advances (2016)
-
"Standardization of Chemical Analytical Techniques for Pyrolysis Bio-oil: History, Challenges, and Current Status of Methods," Bioproducts & Biorefining (2016)
-
"In-Depth Investigation on Quantitative Characterization of Pyrolysis Oil by 31P NMR," RSC Advances (2016)
-
"Determination of Carbonyl Groups in Pyrolysis Bio-oils Using Potentiometric Titration: Review and Comparison of Methods," Energy & Fuels (2016)
-
"Recent Advances in Heterogeneous Catalysts for Bio-Oil Upgrading via 'Ex-Situ Catalytic Fast Pyrolysis': Catalyst Development through the Study of Model Compounds," Green Chemistry (2014)
Additional Publications and Data
View all NREL Publications for Jack Ferrell.
Contact Information
15013 Denver West Parkway, MS 3317
Golden, CO 80401
E-mail: jack.ferrell@nrel.gov | Phone: 303-384-7777
Please contact me with any research questions or ideas for collaborations. Please do NOT contact me regarding jobs at NREL—see instead information on NREL Careers for current opportunities.
Share

