Experimental and Computational Kinetics for Fuel Combustion
NLR works to form a molecular-level understanding of fuel property impacts on engine efficiency through fundamental kinetics and high-fidelity combustion simulations.
Our research supports the development of advanced combustion simulations by experimentally exploring fuel chemical reaction mechanisms and developing kinetics models.
We leverage high-performance computing resources to allow for the rapid development and market entry of high-performance on- and off-road transportation fuels that are compatible with existing engines, enabling the reimagination of future efficient engine design.
Combustion Kinetics Models
In collaboration with partners at Lawrence Livermore National Laboratory, we develop kinetics models for new fuels based on flow reactor data as well as data produced by other partners. These models predict critical features of combustion, such as ignition delay and flame speed. Our goal is to create models that can be coupled with computational fluid dynamics (CFD) simulations, and these must be significantly reduced in size from fully detailed kinetics models. We specialize in producing accurate reduced models for use in CFD simulations.
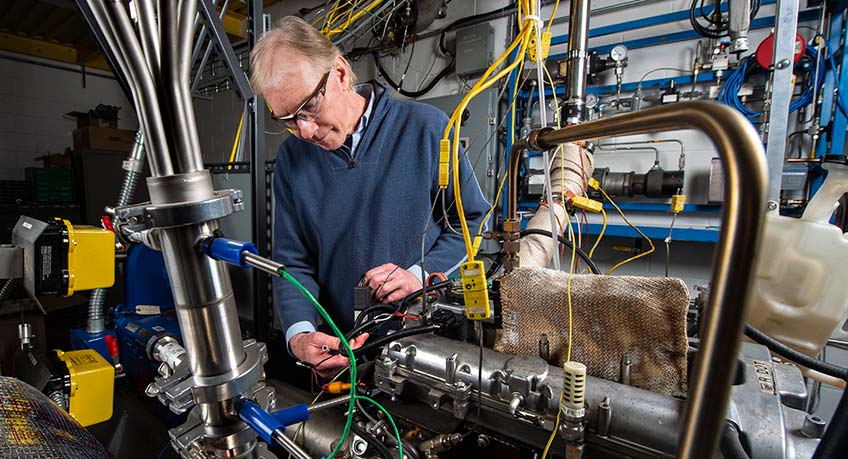
Laminar Flow Reactor for Kinetic Simulations
For the development of accurate kinetic simulations, NLR uses a laminar flow reactor to study mechanisms of fuel autoignition, combustion, and soot precursor formation by quantification of stable intermediates. The flow reactor can operate at up to 1200K and 10 bar and allows us to study the organic chemistry of combustion. The reactor consists of a:
- System for metering gases and fuels
- Stainless steel with inert coating
- Gas sampling and analysis system.
This system allows identification and quantification of the many intermediate chemical species that form during combustion over a range of temperatures, pressures, and fuel to air ratios.
Publications
Read about related studies and browse all NLR publications on combustion mechanism and kinetics R&D.
Chemical Kinetics Investigation of Dibutyl Ether Isomers Oxidation in a Laminar Flow Reactor, Energy & Fuels (2024)
High-Pressure Rate Rules for Ether Alkylperoxy Radical Isomerization, Journal of Physical Chemistry A (2024)
An Experimental and Chemical Kinetic Modeling Study of 4-Butoxyheptane Combustion, Combustion and Flame (2024)
Development of a Diesel Surrogate for Improved Autoignition Prediction: Methodology and Detailed Chemical Kinetic Modeling, Applications in Energy and Combustion Science (2023)
A Comparison Between Methyl Hexanoate and Methyl 3-Hydroxyhexanoate Reactivity: Flow Reactor Experiment and Kinetic Study, Combustion and Flame (2023)
Chemical Kinetic Basis of Synergistic Blending for Research Octane Number, Fuel (2022)
Elucidating the Chemical Pathways Responsible for the Sooting Tendency of 1 and 2-Phenylethanol, Proceedings of the Combustion Institute (2021)
Contact
Share
Last Updated Dec. 6, 2025
