
Daniel Ruddy
Inorganic & Materials Chemist
Dan.Ruddy@nrel.gov
303-384-6322
 https://orcid.org/0000-0003-2654-3778
https://orcid.org/0000-0003-2654-3778
Research Interests
Inorganic chemistry and catalysis
New synthetic pathways to functional materials
Renewable fuels production and processes
Biomass conversion catalysis
Areas of Expertise
Dan received a Ph.D. degree in Inorganic Chemistry from the University of California, Berkeley in 2008. His doctoral research combined synthetic molecular and materials chemistry with detailed characterization and performance testing of novel heterogeneous catalysts. He then worked on a variety of catalyst development projects at the Dow Chemical Company in the Renewable Feedstocks & Process Catalysis Group before joining the National Renewable Energy Laboratory (NREL) in 2010. Dan's research at NREL integrates the synthesis and characterization of functional molecules and materials to enable advanced renewable fuels production and related energy technologies. Areas of expertise include:
-
Inorganic molecular and materials synthesis and characterization
-
Molecular precursor approaches to nano- and meso-scale materials
-
Compositional and morphological control of materials
-
Surface chemistry
-
Catalysis science
-
Heterogeneous catalyst design and synthesis
-
Zeolite synthesis and modification
-
-
Electrocatalysis and photocatalysis
-
In-situ and operando characterization techniques
Education
Ph.D., Chemistry, University of California, Berkeley, 2008
B.S., Chemistry, Lafayette College, 2003
Professional Experience
Staff Scientist, National Bioenergy Center, NREL, 2011–present
Post-Doctoral Researcher - NREL, Chemistry and Nanoscience Center, 2010–2011
Senior Chemist, The Dow Chemical Company, 2008–2009
Patents
-
Catalysts and methods for converting carbonaceous materials into fuels, U.S. Patent No. 9,714,387 (2017)
-
Catalysts and methods for converting carbonaceous materials into fuels, U.S. Patent No. 9,796,931 (2017)
-
Catalysts and methods for converting carbonaceous materials into fuels, U.S. Patent No. 9,803,142 (2017)
-
Metal phosphide catalysts and methods for making the same and uses thereof, U.S. Patent No. 9,636,664 (2017)
-
Magnesium-based methods, systems, and devices, U.S. Patent No. 9,843,080 (2017)
Featured Publications
-
"Exploring Low-Temperature Dehydrogenation at Ionic Cu Sites in Beta Zeolite To Enable Alkane Recycle in Dimethyl Ether Homologation," ACS Catalysis (2017)
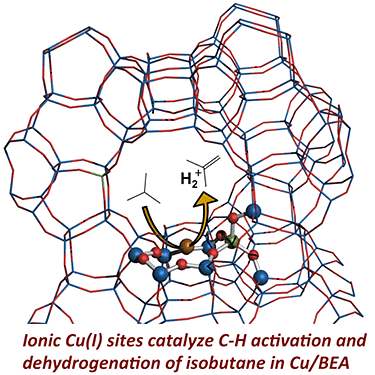
-
"Synthesis of α-MoC1-x Nanoparticles with a Surface-Modified SBA-15 Hard Template: Determination of Structure-Function Relationships in Acetic Acid Deoxygenation," Angewandte Chemie, International Edition (2016)
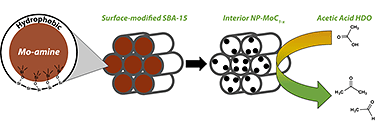
-
"Experimental and Computational Investigation of Acetic Acid Deoxygenation over Oxophilic Molybdenum Carbide: Surface Chemistry and Active Site Identity," ACS Catalysis (2016)
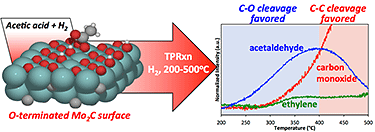
-
"A Facile Molecular Precursor Route to Metal Phosphide Nanoparticles and Their Evaluation as Hydrodeoxygenation Catalysts," Chemistry of Materials (2015)
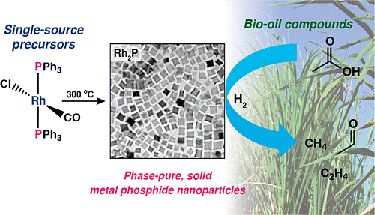
-
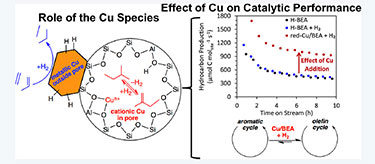
"Conversion of dimethyl ether to 2,2,3-trimethylbutane over a Cu/BEA catalyst: Role of Cu sites in hydrogen incorporation," ACS Catalysis (2015)
-
"Structure-Function Relationships for Electrocatalytic Water Oxidation by Molecular [Mn12O12] Clusters," Inorganic Chemistry (2015)
![Structure-function relationships: at least 1e oxidation and ligand induced distortion at Mn. Series of Mn12O12(OAc)16–xLx(H2O)4 molecular clusters (L = acetate, benzoate, benzenesulfonate, diphenylphosphonate, dichloroacetate) were electrocatalytically investigated as water oxidation electrocatalysts on a fluorine-doped tin oxide glass electrode. Four of the [Mn12O12] compounds demonstrated water oxidation activity at pH 7.0 at varying overpotentials (640–820 mV at 0.2 mA/cm2) and with high Faradaic efficiency (85–93%). For the most active complex, more than 200 turnovers were observed after 5 minutes.](/bioenergy/assets/images/ruddy_graphic2.jpg)
Additional Publications
-
"Thermodynamic Stability of Molybdenum Oxycarbides Formed from Orthorhombic Mo2C in Oxygen-Rich Environments," The Journal of Physical Chemistry C (2018)
-
"Late-Transition-Metal-Modified β‐Mo2C Catalysts for Enhanced Hydrogenation during Guaiacol Deoxygenation," ACS Sustainable Chemistry and Engineering (2017)
-
"High-Throughput Continuous Flow Synthesis of Nickel Nanoparticles for the Catalytic Hydrodeoxygenation of Guaiacol," ACS Sustainable Chemistry and Engineering (2017)
-
"An investigation into support cooperativity for the deoxygenation of guaiacol over nanoparticle Ni and Rh2P," Catalysis Science and Technology (2017)
-
"Evaluation of Silica-Supported Metal and Metal Phosphide Nanoparticle Catalysts for the Hydrodeoxygenation of Guaiacol Under Ex Situ Catalytic Fast Pyrolysis Conditions," Topics in Catalysis (2016)
-
"Organometallic model complexes elucidate the active gallium species in alkane dehydrogenation catalysts based on ligand effects in Ga K-edge XANES," Catalysis Science and Technology (2016)
-
"Role of the Support and Reaction Conditions on the Vapor-Phase Deoxygenation of m‐Cresol over Pt/C and Pt/TiO2 Catalysts," ACS Catalysis (2016)
-
"Mixed alcohol dehydration over Brønsted and Lewis acidic catalysts," Applied Catalysis A (2016)
-
"Synthesis, Optical, and Photocatalytic Properties of Cobalt Mixed-Metal Spinel Oxides Co(Al1-xGax)2O4," Journal of Materials Chemistry A (2015)
-
"Recent advances in heterogeneous catalysts for bio-oil upgrading via 'ex situ catalytic fast pyrolysis': Catalyst development through the study of model compounds," Green Chemistry (2014)
-
"Deactivation and Stability of K-CoMoSx Mixed Alcohol Synthesis Catalysts," Journal of Catalysis (2014)
-
"Surface Chemistry Exchange of Alloyed Germanium Nanocrystals: A Pathway Toward Conductive Group IV Nanocrystal Films," The Journal of Physical Chemistry Letters (2013)
-
"Non-aqueous Thermolytic Route to Oxynitride Photomaterials using Molecular Precursors Ti(OtBu)4 and N≡Mo(OtBu)3," Journal of Materials Chemistry A (2013)
-
"Control of PbSe Quantum Dot Surface Chemistry and Photophysics Using an Alkylselenide Ligand," ACS Nano (2012)
-
"Size and Bandgap Control in the Solution-Phase Synthesis of Near-Infrared-Emitting Germanium Nanocrystals," ACS Nano (2010)
View all NREL Publications for Daniel Ruddy.
Share
