Scientists Offer Companies a Novel Chemistry for Greener Polyurethane
Groundbreaking Formulation Enables Renewable, Nontoxic Polyurethane—a Potential Drop-In Solution for Companies’ Products
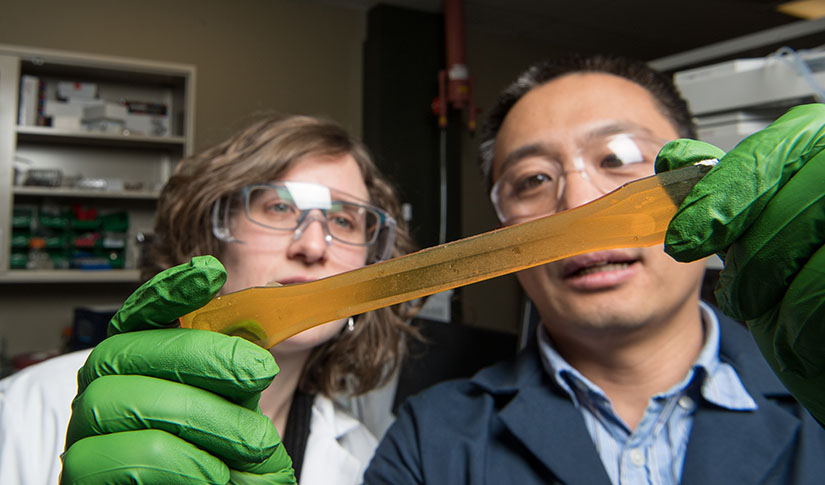
Without it, the world might be a little less soft and a little less warm. Our recreational clothing might shed less water. The insoles in our sneakers might not provide the same therapeutic arch support. The wood grain in finished furniture might not “pop.”
Indeed, polyurethane—a common plastic in applications ranging from sprayable foams to adhesives to synthetic clothing fibers—has become a staple of the 21st century, adding convenience, comfort, and even beauty to numerous aspects of everyday life.
The sheer versatility of the material, which is currently made largely from petroleum byproducts, has made polyurethane the go-to plastic for a range of products. Today, more than 16 million tons of polyurethane are produced globally every year.
“Very few aspects of our lives are not touched by polyurethane,” reflected Phil Pienkos, a chemist who recently retired from the National Renewable Energy Laboratory (NREL) after nearly 40 years of research.
But Pienkos—who built a career researching new ways for producing bio-based fuels and materials—said there is a growing push to rethink how polyurethane is produced.
“Current methods largely rely on toxic chemicals and non-renewable petroleum,” he said. “We wanted to develop a new plastic with all the useful properties of conventional poly but without the costly environmental side effects.”
Was it possible? Results from the laboratory give a resounding yes.
Through a novel chemistry using nontoxic resources like linseed oil, waste grease, or even algae, Pienkos and his NREL colleague Tao Dong, an expert in chemical engineering, have developed a groundbreaking method for producing renewable polyurethane without toxic precursors.
It is a breakthrough with the potential to green the market for products ranging from footwear, to automobiles, to mattresses, and beyond.
But to grasp the sheer weight of the accomplishment, it is helpful to look back at how the scientific advance came about, a story that wanders from the chemical fundamentals of conventional polyurethane, into the algae lab where an idea for a new chemistry first emerged, and winds its way to new corporate partnerships that set the stage for a promising future of commercialization.

A Question of Chemistry
When polyurethane first became commercially available in the 1950s, it quickly grew in popularity for use in numerous products and applications. That was in no small part due to the dynamic and tunable properties of the material, as well as the availability and affordability of the petroleum-based components used to make it.
Through a clever chemical process using polyols and isocyanates—the basic building blocks of conventional polyurethanes—manufacturers could tailor their formulations to produce a stunning variety of polyurethane materials, each with unique and useful properties.
Producing from a long-chain polyol, for example, might yield flexible foams for a pillow-soft mattress. Another formulation might yield a rich liquid that, when spread on furniture, both protects and reveals the inherent beauty of wood grain. A third batch might include carbon dioxide (CO2) to expand the material, producing a sprayable foam that dries into rigid and porous insulation, perfect for holding heat in a home.
“That’s the beauty of isocyanate,” said Dong when reflecting on conventional polyurethane, “its ability to form foams.”
But Dong said that isocyanates bring significant downsides, too. While these chemicals have fast reactivity rates, making them highly adaptable to many industry applications, they are also highly toxic, and they are produced from an even more toxic feedstock, phosgene. When inhaled, isocyanates can lead to a range of adverse health effects, like skin, eye, and throat irritation, asthma, and other serious lung problems.
“If products containing conventional polyurethanes are burned, those isocyanates are volatilized and released into the atmosphere,” Pienkos added. Even simply spraying polyurethane for use as insulation, Pienkos said, can aerosolize isocyanate, requiring workers to take careful precautions to protect their health.
To try and tackle these and other issues—such as reliance on petrochemicals—scientists from labs around the world have begun looking for new ways to synthesize polyurethane using bio-based resources. But these efforts have largely had mixed results. Some lacked the performance needed for industry applications. Others were not completely renewable.
The challenge to improve polyurethane, then, remained ripe for innovation.
“We can do better than this,” thought Pienkos five years ago when he first encountered the predicament. Energized by the opportunity, he joined with Dong and Lieve Laurens, also of NREL, on a search for a better polyurethane chemistry.
Rethinking the Building Blocks of Polyurethane
The idea grew from a seemingly unrelated laboratory problem: lowering the cost of algae biofuels. As with many conventional petrochemical refining processes, biofuel refiners look for ways to use the coproducts of their processes as a source of revenue.
The question becomes much the same for algae biorefining. Can the waste lipids and amino acids from the process become ingredients for a prized recipe for polyurethane that is both renewable and nontoxic?
For Dong, answering the question at the basic chemical level was the easy part—of course they could. Scientists in the 1950s had shown it was possible to synthesize polyurethane from non-isocyanate pathways.
The real challenge, Dong said, was figuring out how to speed up that reaction to compete with conventional processes. He needed to produce polymers that performed at least as well as conventional materials, a major technical barrier to commercializing bio-based polyurethanes.
“The reactivity of the non-isocyanate, bio-based processes described in the literature is slower,” Dong explained. “So we needed to make sure we had reactivity comparable to conventional chemistry.”
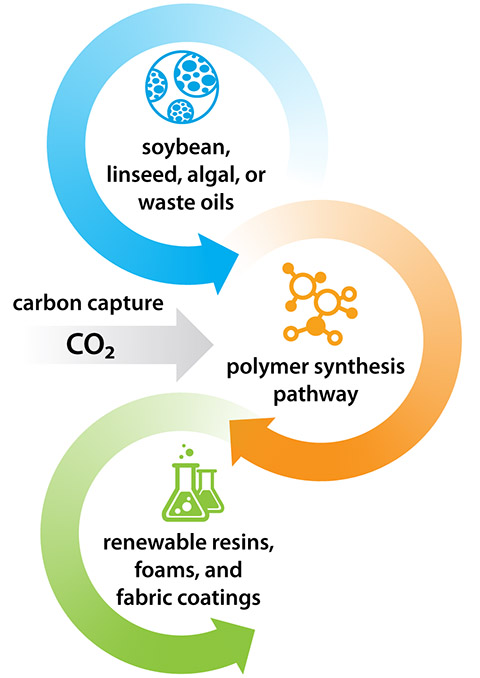
NREL’s process overcomes the barrier by developing bio-based formulas through a clever chemical process. It begins with an epoxidation process, which prepares the base oil—anything from canola oil or linseed oil to algae or food waste—for further chemical reactions. By reacting these epoxidized fatty acids with CO2 from the air or flue gas, carbonated monomers are produced. Lastly, Dong combines the carbonated monomers with diamines (derived from amino acids, another bio-based source) in a polymerization process that yields a material that cures into a resin—non-isocyanate polyurethane.
By replacing petroleum-based polyols with select natural oils, and toxic isocyanates with bio-based amino acids, Dong had managed to synthesize polymers with properties comparable to conventional polyurethane. In other words, he had developed a viable renewable, nontoxic alternative to conventional polyurethane.
And the chemistry had an added environmental benefit, too.
“As much of 30% by weight of the final polymer is CO2,” Pienkos said, adding that the numbers are even more impressive when considering the CO2 absorbed by the plants or algae used to create the oils and amino acids in the first place.
CO2, a ubiquitous greenhouse gas, is often considered an unfortunate waste product of various industrial processes, prompting many companies to look for ways to absorb it, eliminate it, or even put it to good use as a potential source of profit. By incorporating CO2 into the very structure of their polyurethane, Pienkos and Dong had provided a pathway for boosting its value.
“That means less raw material per pound of polymer, lower cost, and a lower overall carbon footprint,” Pienkos continued. “It looks to us that this offers remarkable sustainability opportunities.”
A Sought-After Renewable Solution Finds Its Commercial Feet
The next step was to see if the process could be commercialized, scaled up to meet the demands of the market.
After all, renewable or not, polyurethane needs to demonstrate the properties that consumers expect from brand-name products. The process to create it must also match companies’ manufacturing processes, allowing them to “drop in” the new material without prohibitively costly upgrades to facilities or equipment.
“That’s why we need to work with industry partners,” Dong explained, “to make sure our research aligns with their manufacturing processes.”
In addition to partial funding by the U.S. Department of Energy’s (DOE's) Bioenergy Technologies Office, in the two short years since Pienkos and Dong first demonstrated the viability of producing fully renewable, nontoxic polyurethane, several companies have already contributed resources and research partnerships in the push for its commercialization.
A 2020 DOE Technology Commercialization Fund award, for example, brought in $730,000 of federal funding to help develop the technology, as well as matching “in kind” cost share from the outdoor clothing company Patagonia, the mattress company Tempur Sealy, and a start-up biotechnology company called Algix.
And Pienkos says companies from other industries have shown preliminary interest, too. “These companies believe there is promise in this,” he said.
Their interest could partly be due to the tunability of Pienkos and Dong’s approach, which lets them, much like conventional methods, create polymers that match industry standards.

“We’ve demonstrated that the chemistry is tunable,” Dong said. “We can control the final performance through our approach.”
By controlling the epoxidation process or amount of carbonization, for example, the process can be suited to meet the performance needs of a product. That may give the outsoles of a pair of running shoes enough flexibility and strength to endure many miles pounding into hot or cold asphalt. Or it may give a mattress a balance of stiffness and support.
“It’s got regulation push. It’s got market pull. It’s got the potential to compete with non-renewables on the basis of cost. It’s got a lower carbon footprint. It’s got everything,” Pienkos said of the opportunities for commercialization. “This became the most exciting aspect of my career at NREL. So, when I retired, I decided that I want to make this real. I want to see this technology actually make it into the marketplace.”
After retiring last April, Pienkos went on to establish a company, Polaris Renewables, to help accelerate the commercialization of the novel polyurethane. So, while he continues with his responsibilities as an NREL emeritus researcher, he is also doing outreach to industry to find additional corporate partners, especially in the fashion industry through the international sustainability initiative Fashion for Good.
“In the fashion industry, customers are demanding sustainability,” he explained. “They will pay something of a green premium if you can demonstrate a lower carbon footprint, better end of life disposition.”
Indeed, for both Pienkos and Dong, the breakthrough in renewable, nontoxic polyurethane has become more than an exciting scientific venture. It offers the world a pathway for products that leave a lighter mark on the environment.
“I think this is a great opportunity to solve the plastic pollution problem,” Dong said. “We need to save our environment, and part of that begins with making plastic renewable.”
Pienkos, too, thinks that a commercial success in this venture could be a catalyst that spurs further growth and further success in bringing renewable, greener products to the market.
“This could be a success story for NREL,” he said. “A success here means a great deal to the world.”
In this case, success might be measured in more than the affordability of the production process or the carbon uptake of the polyurethane chemistry. In a world with NREL’s renewable, nontoxic polyurethane, success might be something we can truly feel in the durability of our clothing, in the comfort our shoes provide, or in the rejuvenation we feel after sleeping on a memory foam mattress.
Last Updated Jan. 22, 2026
