Photovoltaics
NLR's photovoltaic (PV) research efforts in chemistry and nanoscience include high-efficiency crystalline, organic, perovskite, and quantum dot solar cells as well as semiconductors.
These activities within the Chemistry and Nanoscience Center are funded by the U.S. Department of Energy Office of Basic Energy Science and Office of Energy Efficiency and Renewable Energy.
High-Efficiency Crystalline Photovoltaics
We are working to increase cell efficiency and reduce manufacturing costs for the highest-efficiency photovoltaic devices involving single-crystal silicon and III-Vs. We are key players in developing low-cost, manufacturable techniques for further increasing the efficiency of advanced silicon cells and continue to be at the forefront of developing the highest-efficiency III-V multijunction cells for both space and high-concentration terrestrial applications. We are also a driving force in two industry-relevant areas: low-cost III-V photovoltaic cells for 1-sun and low-concentration terrestrial applications and very high-efficiency silicon-based tandem cells.
Organic Photovoltaics
We develop and apply new absorber, contact, and barrier materials to advance the device performance and lifetime of organic solar cells. We have had an ongoing focus on developing new contact materials and device architectures. We continue to develop new electron/hole contact layers for increased lifetime and device performance, and tools for the intelligent design and synthesis of new absorber/donor materials. And we have developed a combinatorial degradation system that allows us to measure lifetime of thin-film devices under light, at different substrate temperatures, with or without filters or under different duty cycles. Learn more about NLR's research on organic PV.
Perovskites
We are seeking to make perovskite solar cells a viable technology by focusing on its efficiency, stability, and scaling. Our research is currently focused in several areas including:
- Ultrahigh-efficiency and low-cost polycrystalline halide perovskite thin-film solar cells
- Electronic energy level alignment at the carbon nanotube/organic metal halide perovskite interface
- Stable perovskite solar cells via chemical vapor deposition.
Learn more about NLR's research on perovskite solar cells.
Hybrid Organic-Inorganic Semiconductors
Hybrid perovskites are researched in the Center for Hybrid Organic-Inorganic Semiconductors for Energy (CHOISE), where the mission is to accelerate the discovery and elucidate the design principles for unprecedented control over emergent properties involving spin, charge, and light-matter interactions, leading to new energy-efficient advanced technologies.
NLR leads CHOISE, which includes partners from Duke University, San Diego State University, SLAC National Accelerator Laboratory, the University of Chicago, the University of North Carolina at Chapel Hill, the University of Toledo, and the University of Utah.
Quantum Dot Solar Cells
Quantum dot solar cells are researched in the Center for Advanced Solar Photophysics (CASP). The CASP effort at NLR has focused on advanced concepts for next-generation PV devices based on multiple exciton generation (MEG) or carrier multiplication, Auger upconversion, as well as multijunction PV and solar luminescent concentrator devices.
CASP focuses on new technology that can surpass the efficiency of current systems. Many of these processes involve the unique photophysics found in quantum-confined colloidal nanocrystals. Thus, efforts also focus on understanding nanocrystal surfaces, transport in quantum dot (QD) arrays, and optoelectronic properties of materials.
MEG is a process similar to impact ionization, where one photon can generate multiple excitons or charge carriers in a material. The photon must have sufficient energy (generally more than twice the bandgap energy) and upon absorption, it creates multiple electrons and holes—which thereby increase the amount of current that the PV device produces compared to conventional solar cells. Colloidal QDs of PbSe were used in the first measurements and devices displaying this effect. Current efforts are aimed at exploiting the dimensionality, surfaces, and interfaces in nanomaterials to further increase the efficiency of the process.
MEG enables devices with power conversion efficiency of 44% compared to conventional photovoltaics where the efficiency is limited to 32% (the Shockley-Queisser limit).
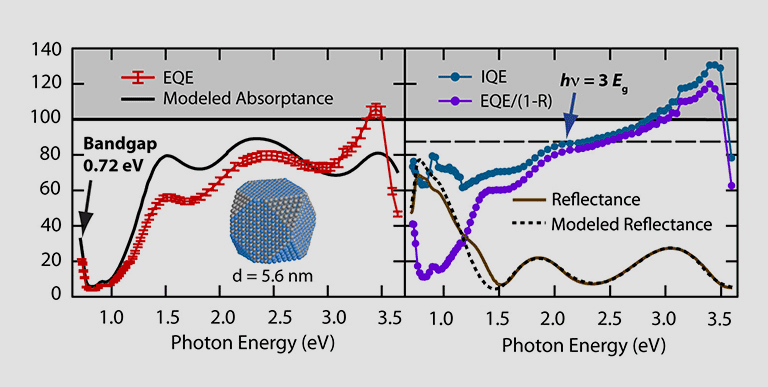
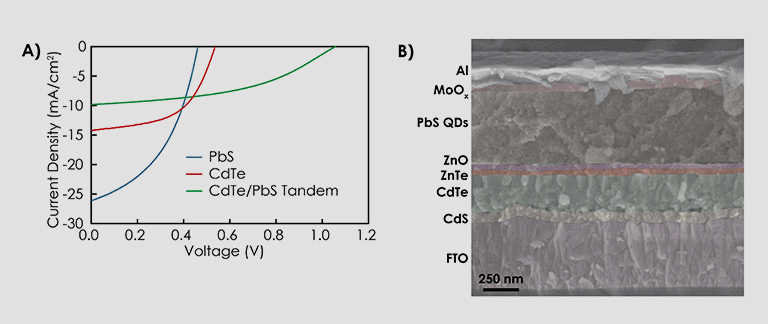
Multijunction photovoltaics effectively split the solar spectrum to enable multiple absorber layers that can convert different parts of the spectrum more efficiently. Within CASP, QDs with tunable bandgap energy offer a unique platform to pair different absorber materials. PbS and PbSe have bandgap energies tunable across the 0.4- to 1.5-eV range and have been combined with solution-processed CdTe in two-terminal tandem configuration.
Cell and Module Performance
We perform current-voltage, quantum efficiency, and other device performance measurements on a range of photovoltaic cell and module technologies—including commercial, developmental, and research samples—for scientists in the photovoltaic industry and at universities.
We have one of only two labs in the world to hold an ISO 17025 accreditation for primary reference cell and secondary module calibration, in addition to accreditation for secondary reference cell calibration under ASTM and IEC standards.
Contacts
The Chemistry and Nanoscience Center is part of the Materials, Chemical, and Computational Science directorate, led by Associate Laboratory Director Bill Tumas.
Share
Last Updated Dec. 6, 2025
