
Research Interests
Understanding and Engineering Multimodular Cellulase Systems
I am interested in the novel mechanisms by which newly discovered multimodular cellulase enzymes such as CelA interact with and degrade crystalline cellulose as well as whole biomass with an aim to design and optimize enhanced cellulase systems for overcoming biomass recalcitrance.
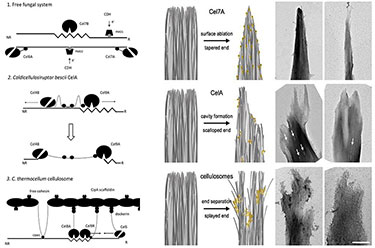
Most fungi and bacteria degrade plant cell walls by secreting free, complementary enzymes that hydrolyze cellulose; however, some bacteria use large enzymatic assemblies called cellulosomes, which recruit complementary enzymes to protein scaffolds. The thermophilic bacterium Caldicellulosiruptor bescii uses an intermediate strategy, secreting many free cellulases that contain multiple catalytic domains. One of these, CelA comprises a glycoside hydrolase family 9 and a family 48 catalytic domain, as well as three type III cellulose-binding modules. In the saccharification of a common cellulose standard, Avicel, CelA outperforms mixtures of commercially relevant exo and endoglucanases. From transmission electron microscopy studies of cellulose after incubation with CelA, we have discovered novel morphological features that suggest that CelA not only exploits the common surface ablation cellulose digestion mechanism driven by general cellulase processivity, but it also excavates extensive cavities into the surface of the substrate. These results suggest that natures repertoire of cellulose digestion paradigms remain only partially discovered and understood.
Plant Cell Wall Engineering and Recalcitrance Reduction
I am interested in the possible uses of glycoside hydrolase enzymes expressed in-planta to reduce plant cell wall recalcitrance. Current methods for introducing exogenous enzymes to biomass chips, as used today, are limited by multi-length scale diffusion barriers, both at the level of the biomass chip and the structure of the cell wall. There are multiple macro-scale and micro-scale factors contribute to the recalcitrance of lignocellulosic feedstock to both thermochemical pretreatment and subsequent enzymatic saccharification. On a micro-scale, important factors include the degree of lignification and the structural heterogeneity and complexity of cell-wall constituents such as cellulose microfibrils and matrix polymers such as xylan. In the past, in-planta expression of enzymes was primarily focused on addressing the enzyme cost problem by proposing to produce glycoside hydrolases (GH) in plants utilizing the plant itself as a bio-factory to produce large quantities of the enzymes, which would be extracted prior to pretreatment and then added back to the pretreated biomass to reduce the overall cost of the enzymatic hydrolysis.
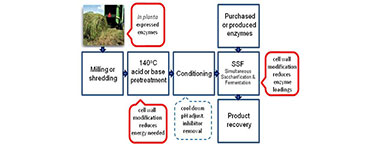
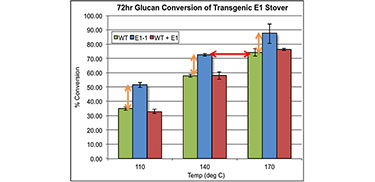
Recently we have discovered that expressing a single endoglucanase in-planta can have profound effects on reducing the overall recalcitrance of the plant cell wall. Our functionally modified model crops were found to have reduced recalcitrance compared to wild type crops while maintaining a normal phenotype. And the effect was non-replicable by just adding back the enzyme after the plant had scenesed. The further advantage of these crops was that it was possible to reduce the thermochemical pre-treatment requirements of the plants significantly, offering large techno-economic advantages by using reduced severity pretreatments. These two factors make this recombinant plant technology an excellent starting point for developing sustainable energy production crops.
Education
-
Ph.D., Pharmacology, University of Colorado Health Sciences Center, 2007
-
B.A., Biochemistry, University of Colorado Boulder, 1999
Professional Experience
-
Senior Scientist, National Renewable Energy Laboratory (NREL), Chemical and Biosciences Center, 2012–present
-
Staff Scientist, NREL, Chemical and Biosciences Center, 2008–2012
-
Post-Doc, NREL, Chemical and Biosciences Center, 2008–2012
Patents
-
"Enzymes for improved biomass conversion," U.S. Patent Application No. 20140017735 (2014)
-
"Multi-Chamber Pretreatment Reactor For High Throughput Screening of Biomass," U.S. Patent Application No. 20100105570 (2010)
-
"Processing Cellulosic Biomass (in planta expression of E1 endoglucanase in maize)," U.S. Patent Application No. 20120040408 (2009)
Featured Publications
-
"High temperature pre-digestion of corn stover biomass for improved product yields," Biotechnology for Biofuels (2014)
-
"Revealing Nature's Cellulase Diversity: The Digestion Mechanism of Caldicellulosiruptor bescii CelA," Science (2013)
-
"In-Planta Expression of A. cellulolyticus Cel5A Endocellulase Reduces Cell Wall Recalcitrance in Tobacco and Maize," Biotechnology for Biofuels (2011)
Additional Publications
-
"The catalytic mechanism and unique low pH optimum of of Caldicellulosiruptor bescii family 3 Pectate Lyase," Acta Crystallographica Section D (2015)
-
"Expression of the Acidothermus cellulolyticus E1 endoglucanase in Caldicellulosiruptor bescii enhances its ability to deconstruct crystalline cellulose," Biotechnology for Biofuels (2015)
-
"Identifying the ionically bound cell wall and intracellular glycoside hydrolases in late growth stage Arabidopsis stems: implications for the genetic engineering of bioenergy crops," Front. Plant Sci. (2015)
-
"Investigating the role of lignin on biphasic xylan hydrolysis during dilute acid and organosolv pretreatment of corn stover," Green Chem. (2015)
-
"New Insights into Microbial Strategies for Biomass Conversion," in Direct Microbial Conversion of Biomass to Advanced Biofuels (2014)
-
"Feedstock Engineering and Biomass Pretreatments: New Views for a Greener Biofuels Process," in Direct Microbial Conversion of Biomass to Advanced Biofuels (2014)
-
"Response to Comment on 'Revealing Nature's Cellulase Diversity: The Digestion Mechanism of Caldicellulosiruptor bescii CelA'", Science (2014)
-
"Enzymatic Hydrolysis of Lignocellulosic Biomass," Bioprocessing of Renewable Resources to Commodity Bioproducts (2014)
-
"Charge Engineering of Cellulases Improves Ionic Liquid Tolerance and Reduces Lignin Inhibition," Biotechnology and Bioengineering (2014)
-
"Basic biological research relevant to feedstock conversion," in Compendium of Bioenergy Crops (2014)
-
"Cel48A from Thermobifida fusca: Structure and site directed mutagenesis of key residues," Biotechnology and Bioengineering (2013)
-
"Impact of alg3 gene disruption on growth, development, pigment production, and protein secretion, and functions of Trichoderma reesei cellobiohydrolases in Aspergillus niger," Fungal Genetics and Biology (2013)
-
"Improving activity of minicellulosomes by integration of intra- and intermolecular synergies," Biotechnology for Biofuels (2013)
-
"The structure and mode of action of Caldicellulosiruptor bescii family 3 pectate lyase in biomass deconstruction," Acta crystallographica. Section D, Biological crystallography (2013)
-
"Sequence, structure, and evolution of cellulases in the glycoside hydrolase family 48," Journal of Biological Chemistry (2012)
-
"Structure and function of the Clostridium thermocellum Cellobiohydrolase A fibronectin(III)-like module repeat: enhancement through stabilization of the catalytic unit," Acta crytallographica (2012)
-
"Identification and Activity Analysis of Transgenic Glycoside Hydrolases Expressed in Plants," in Methods in Molecular Biology (2012)
-
"Bioprospecting metagenomics of decaying wood: mining for new glycoside hydrolases," Biotechnology for Biofuels (2011)
-
"Structure of a fibronectin type (III)-like module from Clostridium thermocellum," Acta crytallographica (2010)
-
"The unique binding mode of the Cellulosomal CBM4 from Clostridium thermocellum Cellobiohydrolase A," J. Mol. Biol. (2010)
-
"Lignocellulose Recalcitrance Screening by Integrated High-Throughput Hydrothermal Pretreatment and Enzymatic Saccharification," Industrial Biotechnology (2010)
-
"Probing the role of N-linked glycans in the stability and activity of fungal cellobiohydrolases by mutational analysis," Cellulose (2009)
-
"Redistribution of Xylan in Maize Cell Walls during Dilute Acid Pretreatment," Biotechnology Bioengineering (2009)
-
"High-Throughput Screening Techniques for Biomass Conversion," Bioenergy Research (2009)
-
"Heterologous expression of glycosyl hydrolases in planta: a new departure for biofuels," Trends Biotechnol. (2008)
-
"Investigation of the Binding Geometry of a Peripheral Membrane Protein," Biochemistry (2005)
View all NREL Publications for Roman Brunecky.
Share
